P RT V RT v p RT Vm - b. The equation of state for a substance provides the additional information required to calculate the amount of work that the substance does in making a transition from one equilibrium state to another along some specified path.

Derive Van Der Waals Equation Of State For Real Gases Brainly In
Independent of the system size it is possible to express it as a function of purely intensive quantities.

Derive equation of state. Use equations of state to calculate departure functions EoS. 5 corresponds to the well known van der Waals equation of state see Section 263 and is obtained by deriving the latter. The Ideal Gas Law.
In both cases for example. And when A and B both are 0 Next State. The Next State of State table will fill by State Equation.
There is an equation connecting these variables the so-called equation of state EOS. An equation of state can be generated by any other equation expressing one of the residual quantities as a function of temperature and volume or pressure. Pv RT2 or for m kg occupying V m3 pV mRT3.
The state postulate claims that any two intensive variables can fix the state of a system therefore we can tabulate all state. DE TdS PdV X i µidNi 1 where µi is the chemical potential associated with an ith species and clearly can be defined as µi E Ni SV. The equation of state is expressed as a functional relationship connecting the various parameters needed to specify.
But the way we did in previous section Differential Equation meeting Matrix was mainly for mathematical manipulation. Introduction to equations of state with some common examples About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features. For example for the Next State of A x must be equals to 1So where x0 you can simply put the Next State of A 0 in the State Table.
For example the following expression of the residual Helmholtz energy. Essentially this shows a derivation of entropy and that a state function can be written as a total derivative dF xy Fxydx Fyxdy. So you can see in the Table given below where x is 0 Next State of A is 0.
It was not easy to extract any practical meaning out of the matrix. The van der Waals Equation of State is an equation relating the density of gases and liquids to the pressure volume and temperature conditions ie it is a thermodynamic equation of state. It was derived in 1873 by Johannes.
The equation 1 is restated introducing the density ˆ NV becoming. To derive the Van Der Waals equation or to deduce the Van Der Waal equation of state for one mole of gas can be turned into an easy process if the right steps are followed. Equation of State 2 The thermodynamic equation with chemical reactions.
Thus our approximate equation of state becomes 8100. Made by faculty at the University of Colorado Boulder Department of Chemical Biological Engineering. The next condition for Next State of A is either A or B is equals to 1 then the Next State of is 1.
Otherwise Next State of A is 0. Introduction to the Virial equation of state. The equation of state relates the pressure p volume V and temperature T of a physically homogeneous system in the state of thermodynamic equilibrium f p V T 0.
The equation of the state for a perfect gas is thus given by the equation. However State Space Modeling is a method to convert aa set of differential equations into a form of matrix. This allows us to write the equation of state in its usual form 269 The above derivation of the ideal gas equation of state is rather elegant.
The steps for derivation of the real gas equation for one of gas are as follows. It can be viewed as an adjustment to the ideal gas law that takes into account the non-zero volume of gas molecules which are subject to an inter-particle attraction. State Space Modeling is also a kind of way to convert a differential equation into a set of matrix equation.
It is certainly far easier to obtain the equation of state in this manner than to treat the atoms which make up the gas as little billiard balls. 1 Since the pressure is an intensive quantity ie. Thermodynamics - Thermodynamics - Equations of state.
Where q is the heat flow w is the work which we define as PdV and δ indicates that heat flow and work are inexact differentials path functions. This equation of state reveals aspects of the nature of swarms particularly when compared with the linear equation of state for an ideal gas where PV Nk_B T. Ideal Gas Law DEFINITION An equation of state relates the molar density or specific molar volume of a fluid ie a vapor or a liquid to the temperature and pressure of the fluid.
2 The LTE requires that X i µidNi 0 3 As an example H e H0 χ H 4 where χH 136 eV is the ionization potential from the ground state of H.
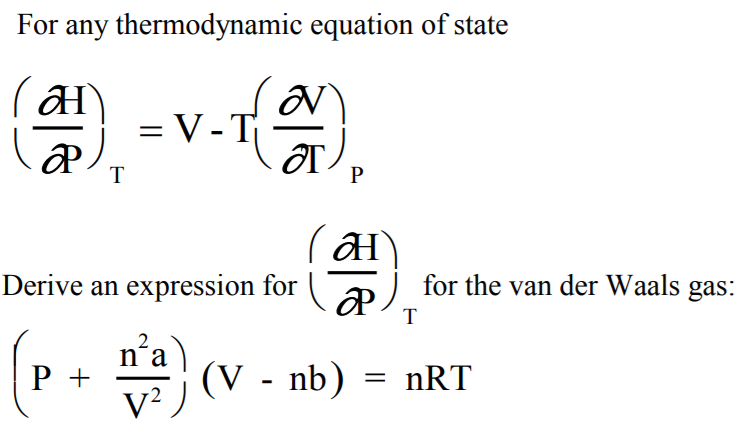
Solved For Any Thermodynamic Equation Of State Th Derive Chegg Com

Solved Beginning With The Van Der Waals Equation Of State Chegg Com

The Statistical Mechanical Derivation Of The Van Der Waals Equation O

Fundamental Relations The Thermodynamic Functions The Molecular Partition Function Using Statistical Thermodynamics Mean Energies Heat Capacities Equation Ppt Download
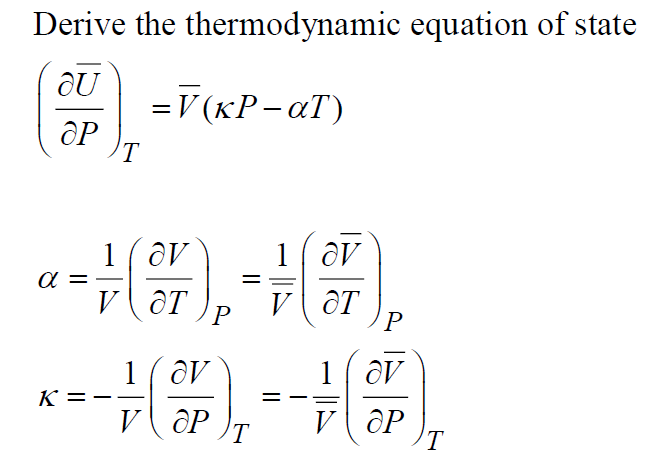
Solved Derive The Thermodynamic Equation Of State Cx Chegg Com
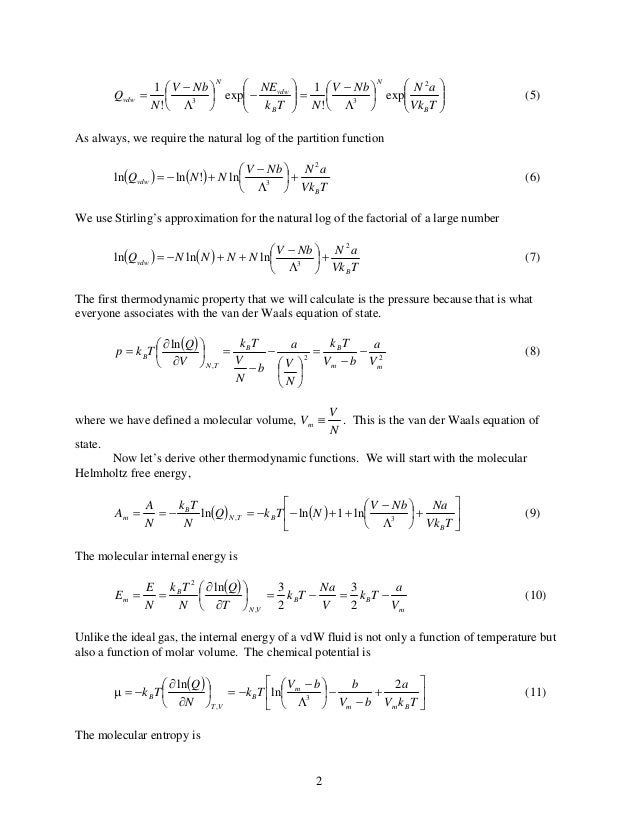
The Statistical Mechanical Derivation Of The Van Der Waals Equation O

Solved Derive The Thermodynamic Equation Of State Derive Chegg Com

Virial Equation Of State Introduction Youtube

Derive Van Der Waals Equation Of Gas Brainly In
The Derivation Of Van Der Waals Equation Of State For Real Gases Gases Continuum Mechanics

Pdf A Modified Form Of The Van Der Waals Equation Of State

The Statistical Mechanical Derivation Of The Van Der Waals Equation O


