Location therefore varies in many cases especially. Major sources are thought to be atmospheric emissions mostly from urban sources although exact locations and the means by which emissions are transformed to acid rain are not entirely clear.

A Study Of Acid Rain Causes And Prevention Technique
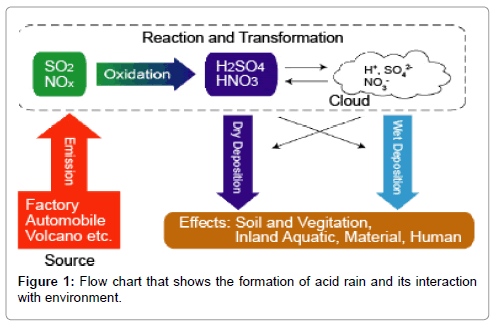
Acid rain is caused by the emissions of sulfur dioxide SO2 and nitrogen oxides NOx.

Methodology of acid rain. Nuclear power hydro-electricity wind energy geothermal energy and solar energy i. Acid rain generally has a lower. The various gases like sulphur dioxide and nitrogen dioxide react with water vapours in presence of.
Due to presence of these acids the pH of the rain water lowered to a value of 24 and this type of raining of lower pH is called acid rain. Acidification of the rainwater is identified by the presence of sulphuric. Mainly nitric oxide NO and nitrogen dioxide NO 2 through the combustion of fossil fuel which react with the water molecules in the atmosphere oxidation to produce H 2 SO 4 and.
Acid rain is also called acid deposition because this term includes other forms of acidic precipitation such as snow. Sulfur dioxide and nitrogen oxides the major sources of acid rain. When harmful gases contained in air pollution react with moisture in the air acid rain can result.
A value of pH below 56 denotes acid rain. Thus the pH is a little below 7 at 56. Reduce the use of air conditioners use lights and fan only when needed and reduce the use of home appliances to.
Is 43 on the pH scale. Acid rain is caused by a chemical reaction that begins when compounds such as sulphur dioxide and oxides of nitrogen are released into the air. Acid rain occurs when the natural PH balance of rain has been changed further towards the acidic side.
Acid rain is made up of water droplets that are unusually acidic because of atmospheric pollution most notably the excessive amounts of sulfur and nitrogen released by cars and industrial processes. Normal rain is actually slightly acidic because it contains CO2. Atmospheric pollutants particularly oxides of sulphur and nitrogen can cause precipitation to become more acidic when converted to sulphuric.
It was first studied in the US in the 1950s. Measures methods to prevent acid rain use of alternative mode of transportation or use more of public transport car pooling system and of course walk more. Acid rain is one of the important environmental threats and occurs due to the presence of certain acids in the atmosphere.
However industrial processes can lower that pH even further the average in the US. Acid rain is a widespread term used to describe all forms of acid precipitation rain snow hail and fog. Ordinary rain is naturally acidic with a pH between 50 and 55.
This essay carried out a survey on the measures various governments are taking to address acid rain. Acid rain contains sulfur dioxide and nitrogen oxides which when they come into contact with water form things like sulfurous acid sulfuric acid and nitrous acid. Acid rain is rain that is more acidic than normal.
Use other sources of electricity ie. Acid rain is actually a type of acid deposition. Acid rain was found to transfer Sulphur and nitrogen compounds through precipitation in the form of rain fog hail or dew.
Acid rain is a complex environmental problem that is hard to define in terms of sources and effects. By William Wanyagah Acid Rain How and Why it occurs effects and methods used to counter Motor Vehicles Effects of Acid Rain Acid Rain may be produced in area but the clouds formed can be carried across great distances by wind. Acid rain results when sulfur dioxide SO 2 and nitrogen oxides NO X are emitted into the atmosphere and transported by wind and air currents.
The SO 2 and NO X react with water oxygen and other chemicals to form sulfuric and nitric acids. Acid rain is caused by emissions of compounds of ammonium carbon nitrogen and sulphur which react with the water molecules in the atmosphere to produce acids. Reduce the use of non.
Lack of long-term records has made it difficult to determine how fast rainfall acidity is changing. PH naturally runs from zero to 14 with zero being the most acidic and 14 being the most alkaline. Generally rain water gets acidic because CO 2 SO 2 and NO 2 present in the atmosphere get dissolved in it forming carbonic acid H 2 CO 3 sulphuric acid H 2 SO 4 and nitric acid HNO 3 respectively.
A PH reading of seven is considered to be neutral on the PH scale. Reducing the effects of Acid Rain by Liming. Powdered limestonelimewater added to water and soil to neutralize acid.
Acid rain was first identified in the 1800s in Sweden. Sulphates can be used for industrial purposes. These then mix with water and other materials before falling to the ground.
These substances can rise very high up into the atmosphere where they mix and react with water oxygen and other chemicals to form more acidic pollutants called acid rain. Acid rains spread and damage involves weather chemistry soil and the life cycles of plants and animals on the land and from acid rain in the water.

Pdf Analysis Of Acid Rain Patterns In Northeastern China Using A Decision Tree Method

Pdf Analysis Of Wet Deposition Acid Rain Determination Of The Major Anionic Constituents By Ion Chromatography

Pdf Science Behind Acid Rain Analysis Of Its Impacts And Advantages On Life And Heritage Structures

Pdf Acid Rain Causes Effects And Control Strategies

Ghg Mandatory Reporting Rule Overview
Https Academic Oup Com Economicpolicy Article Pdf 5 11 297 7094693 Economicpolicy5 0297 Pdf

Pdf Acid Rain And Its Ecological Consequences
Pdf Acid Rain Is A Local Environment Pollution But Global Concern
Mechanisms And Effects Of Acid Rain On Environment

Sunflowers And Acid Rain Talent 21 Project By Gavin Henderson Date Period Ppt Download
