Incubate the plates and count the colonies on both sides of the medium. The application of GMP for pharmaceutical packaging materials helps ensure that these materials meet the needs and requirements of the pharmaceutical industry.

Packaging Good Manufacturing Practices Gmps For Medicinal Products
The bottle can be checked to a preselected pressure level and the test.

Microbiological testing of primary packaging material. It should pass the specifications of tests before it reached the local markets and made available to the consumers of product. The goal of the packaging material is to protect the contents against external factors such as humidity light oxygen or temperature variations. 53 After every one year the approved primary packaging material shall be retested against test parameters mentioned on the respective specification along with microbiological testing.
Primary packaging materials are those that are in direct contact with the product 1. The realization of GMP principles in production and control of primary packaging materials within organizations is of great importance for the safety of a patient using the medicinal product because of their direct product contact. Techniques used are GC-MSMS HS-GC-MS LCMS.
A specified area of the packaging material is cut and placed on a solid agar medium in a petri dish and over laid with medium. Collect 10 gm sample in case of aluminum foil and PVC sheet as per SOP for sampling packing material. Internal Bursting Pressure Test- The most common instrument used is American glass research increment pressure tester.
This technique is for the non absorbent packaging material such cardboard plastics and aluminum foil. The legislative authority has defined in the Pharmacopoeia which ingredients are allowed to be used and has also set limit values if they are necessary. 511 Collect 10 units each of vials rubber stopper and seals and bottle randomly.
Microbiological Testing of Primary Packaging Material. I sanitation tests ie. 52 The primary packaging materials shall be tested for microbiological limit testing as per GTP No.
These tests are applicable to all articles made of plastic that have an even surface and that can thus be easily cleaned for example polyurethane. One of the most important activities for. The microbial contamination introduced through primary packaging needs to be addressed for certain products such as liquid dosage forms or inhalers.
The testing of packaging materials is almost requirement for any pharmaceutical industry. The use of antimicrobial active substances in the primary packaging rather than in the drug product itself could be an attractive option. This test formally known as the Microbial Limits Test determines the bioburden of the product and also if objectionable organisms are present.
513 Bring the sample to microbiology lab for testing. Microbiological Specifications Microbiological criteria. We offer tests like hydrolytic resistance glass grains test surface glass test surface etching test chemical and physicochemical tests Ink Gum migration studies and delamination studies.
Many primary packaging materials can be tested using the membrane filtration method. Microbiological Guidelines Microbiological criteria which provide advice to food manufacturers about acceptable or expected microbial levels when the food production process is under control when applying best practices. On May 1 2009 the harmonized test method became effective requiring manufacturers to meet the new guidelines set forth in it.
The test bottle is filled with water and placed inside the test chamber. I know in BRC standard or many International standard not request about Microbiological test on packaging but some standard request Testing on packaging as Not detect pathogen on packaging I think it maybe come from contaminantion by human or worker during processes or in finished product It cant grow on package but can live on package and will growing after contact food. 512 Use clean and sterilized sampling devices to avoid external contamination.
Thecost of material of a package should be as low as possible without compromising the quality of product. Testing of packaging materials for microbial quality. The material of a package affects quality stability and efficacy of drug product.
The chapter presents a scientific approach where the criterion is defined according to the route of application and the surface of the primary packaging in contact with the amount of drug. Primarypackaging acceptance criteria are given for the food industry. The type of test.
Production All operations involved in the preparation of a pharmaceutical prod-uct from receipt of the starting materials through processing and packaging to. A scaling head is applied and the internal pressure automatically raised by a series of increments each of which is held for a set of time. Packaging process All operations including filling and labelling that a bulk product has to undergo in order to become a finished product 1.
P-080-T in case of only first three consecutive consignments from the Vendor. The following microbiological measurements were determined. The resistance of a plastic product to bacteria and fungi microbes can be determined using these tests which support food packaging cosmetics packaging and many other industries and products.
Microbiological Testing is a mandatory requirement to ensure the quality of the packaging material which has a direct impact on the quality of a drug. Packaging materials such as blister packs infusion bags plastic containers and plastic bags that are used as primary packaging for medicinal products must be checked for their quality. To accomplish this Microbial Examination of Nonsterile Products is performed.
This Guideline supersedes the Guideline on Plastic Primary Packaging Materials published in the Rules Governing Medicinal Products 3AQ10a and addresses the information on plastic materials being used as immediate packaging for active substances and medicinal products to be submitted in marketing authorisation applications. Packaging material testing services are performed as per USP 660 6611 6612 andor customer requirements. Environmental swabs from hands of workers and machines equipment to measure the bacteriological condition of.
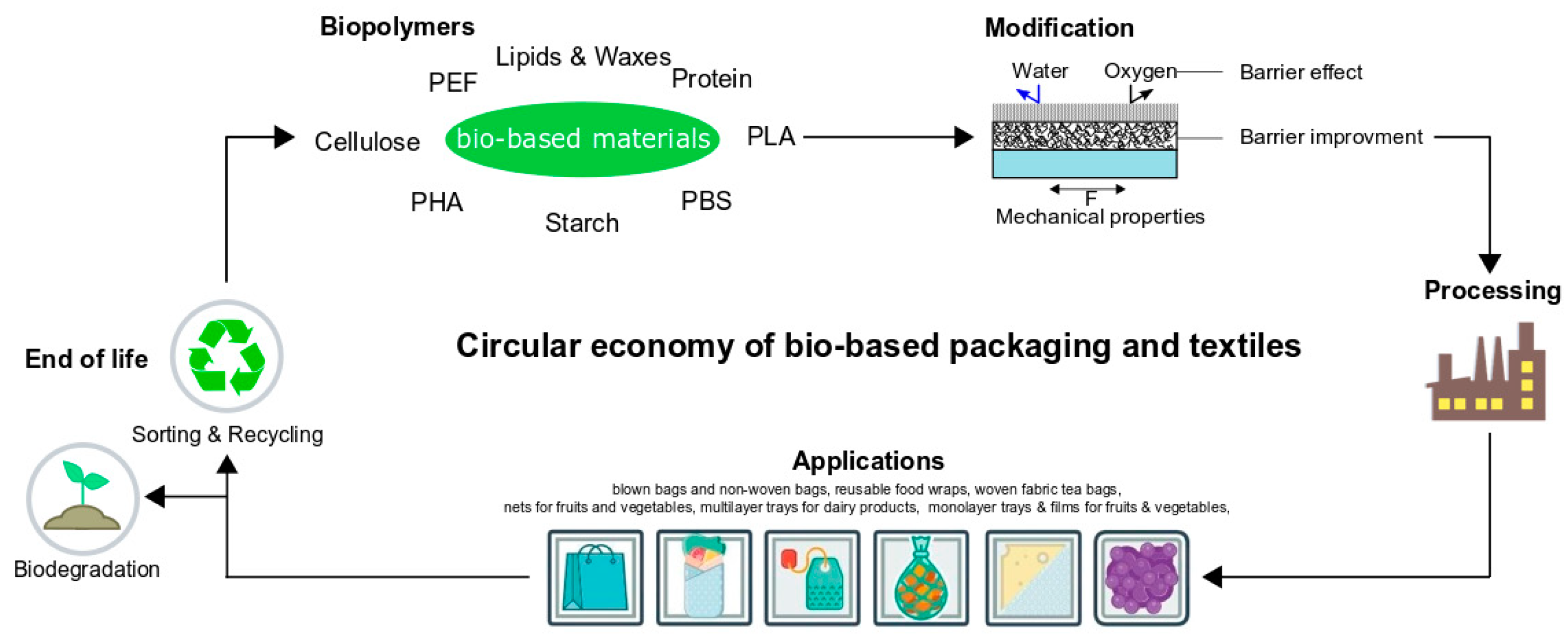
Polymers Free Full Text Bio Based Packaging Materials Modifications Industrial Applications And Sustainability Html

Microbiological Testing Of Primary Packaging Material Askpharmatutor

Food Packaging Paper And Board Testing At Campden Bri

Microbiological Testing Of Primary Packaging Material Askpharmatutor
Https Onlinelibrary Wiley Com Doi Pdf 10 1002 9781119356196 Ch6
Https Onlinelibrary Wiley Com Doi Pdf 10 1002 9781119356196 Ch6

Quality Control Of Packaging Materials Pharmastate Blog

Package Development Of Pharmaceutical Products Aspects Of Packaging Materials Used For Pharmaceutical Products Sciencedirect
Sop For Bioburden Test Of Packing Materials In Microbiology Laboratory Pharma Dekho
Http Pharmaquest Weebly Com Uploads 9 9 4 2 9942916 Hitesh Pharmaceutical Packaging Component And Evaluation Pdf

Live Online Training Reduced Sampling Reduced Testing Eca Academy

Characterization Of Surface Properties Of Glass Vials Used As Primary Packaging Material For Parenterals Sciencedirect

Gmp Logfile Lead Article Gmp Verlag Logfile No 09 2016 Specifications For Packaging Materials
