Calgon is the trade name given to a. Temporary hardness or Carbonate hardness Permanent hardness or Non-carbonate Temporary hardness in water is caused due to the presence of bicarbonates of calcium and magnesium and boiling or addition of lime can easily remove this.
How To Remove Permanent Hardness From Water Quora
Permanent hardness of water can be removed by adding A Na2CO3 B K C CaOClCl D Cl2.
Permanent hardness of water can be removed by addition of. Treating the Water with Washing Soda In this method we add washing soda ie Na2CO3 to the hard water. The addition of Calgon to hard water causes the calcium and magnesium ions of hard water to displace sodium ions from the anion of Calgon. Permanent hardness can be removed by the addition of a.
Sodium carbonate N a2 C O3 is also known as washing soda. These can get into the water when it comes into contact with limestone and other rocks that contain calcium compounds. A Chlorine b Washing Soda c Potassium Permanganate d Bleaching Powder.
Permanent hardness is hardness that cannot be removed by softening. Permanent hardness is caused due to the presence of sulphates chlorides and nitrates of calcium and magnesium. In this method lime Ca OH 2 and sodium carbonate Na 2 CO 3 or soda ash are used to remove permanent hardness from water.
Hardness in water is caused by dissolved magnesium ions and calcium ions. The key difference between temporary and permanent hardness of water is that temporary hardness of water can be removed by boiling the water whereas permanent hardness cannot be removed by boiling. In this case the hardness in water can be removed by boiling the water.
Hardness can be removed by adding sodium carbonate washing soda or by passing the water through an ion-exchange column. Permanent hardness can be removed by the addition of washing soda commonly known as sodium carbonate. Permanent hardness of water can be removed by the following methods.
Temporary hardness is hardness that can be removed by softening. Permanent hardness of water can be removed by adding. The hardness due to the presence of chloride and sulphate salts of calcium and magnesium is known as the permanent hardness of the water.
Washing soda is used to remove the permanent hardness of the water. Therefore we need to use another method to remove permanent hardness from water such as the addition of using a water softener or using an ion-exchange column. Give Balanced Equations for the Same.
The presence of magnesium and calcium carbonates in water makes it temporarily hard. 10 H 2 O addition of sodium polymetaphosphate calgon N a P O 3 n or by ion exchange method. None of these 32.
When we boil water the soluble salts of Mg HCO 3 2 is converted to Mg OH 2 which is insoluble and hence gets precipitated and is removed. Temporary hardness can be removed by boiling or by addition of C a O H 2. After filtration the water we get is soft water.
Both Temporary and Permanent Hardness in Water Can Be Removed by Addition of Washing Soda. We use certain chemical methods to remove the permanent hardness of water which are. Related Questions on General Science The tree which sends down roots from its branches to the soil is known as.
Check Answer and Solution for above ques. Permanent hardness is non-carbonate hardness which is present when the total alkalinity of the water is higher than the total hardness of the water expressed as calcium carbonate. Permanent hardness can be removed by the addition of washing soda N a 2 C O 3.
Permanent hardness of water may be removed by the addition of___________. This results in the removal of calcium and magnesium ions from hard water in the form of a complex with Calgon. It can remove temporary and permanent hardness from water.
Temporary hardness can be removed by boiling or by addition of Ca OH 2. It combines with chloride salts of calcium and magnesium present in the water to form compounds. Pond water well water Temporary hardness of water may be removed by adding calcium hydroxide calcium carbonate calcium chloride sodium bicarbonate Permanent hard water may be softened by passing it through sodium silicate sodium bicarbonate sodium hexametaphosphate sodium phosphate.
Which of the following chemical is sometimes added in the process of coagulation and flocculation.
How To Remove Permanent Hardness From Water Quora
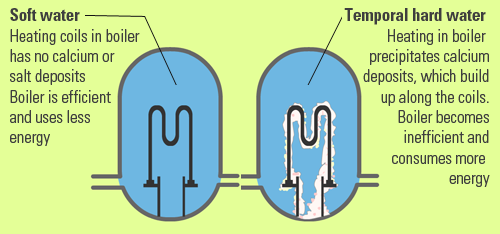
What Is Hard Water And Why Do We Need To Remove Water Hardness Steemit

Permanent Hardneness Of Water Can Be Removed By Chemical Means Find 5 Answers Solutions Learnpick Resources
Removal Of Hardness Of Water Notes Videos Qa And Tests Grade 8 Science Some Useful Chemicals Kullabs

Water Hardness Special Treatment Water Hardness Special Treatment Ppt Video Online Download

Water A Guide For Gcse Students 2010 Knockhardy

Which Of The Chemicals Used To Soften Hard Water

Hard Water L O To Know What Makes Water Hard And The Pros And Cons Associated With This Ppt Download

By Adding Which Of The Following Process Permanent Hardness Of Wa
Removal Of Hardness Of Water Notes Videos Qa And Tests Grade 8 Science Some Useful Chemicals Kullabs

Water The Universal Solvent Introduction Ppt Video Online Download


