So clearly will gain a proton if the pH is below 1 say and it will lose a proton if the pH is above 10. 4243 In our previous study we prepared an amphoteric.

Solubility Ph Profiles Of Some Acidic Basic And Amphoteric Drugs Sciencedirect
H X 2 A K w.
Ph of amphoteric solution. However they did not report the thermo-responsive behavior of the amphoteric copolymers4243 In our previous study we prepared an amphoteric statistical copolymer from cationic. Another interesting case worth of addressing here is calculation of pH for amphiprotic substance HA- present in the solution of acidic salts. PHresultant solution pHNaOH that is 1062 127 Therefore we are reasonably confident that our answer is plausible.
H 3 O 2 K 1 K 2 H 3 O 452 x 10-9 pH 835. 11 12 These SLAA solutions exhibit alkaline pH values in the range of 911 and do not require. Observe the color of the precipitate.
2 H A X H X 2 A A X 2. Similar calculations can be done for any amphiprotic substance. The is possible with 2 simplifying conditions.
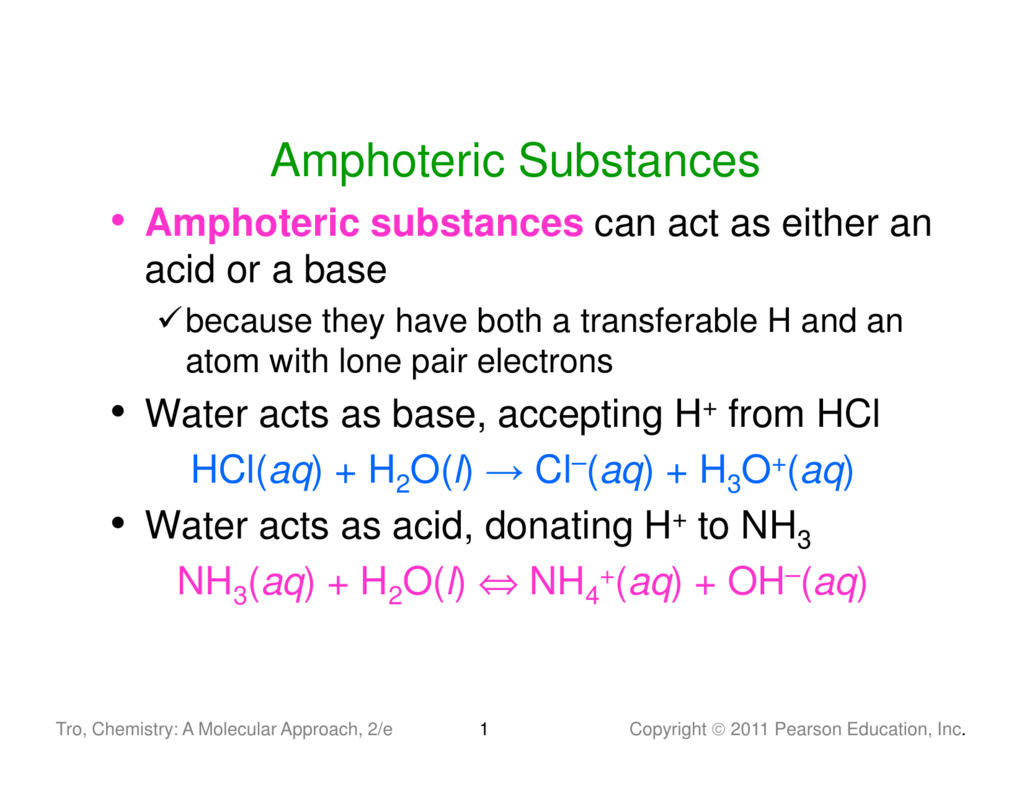
The effects of pH and ionic strength on the wettability of amphoteric surfaces have already been experimentally investigated for titania TiO 2 surfaces coated with a thin silane layer octadecyltryhydrosilane OTHS in a wide range of pH. The concentration of oxonium resp. If you ever to a titration of such amphoteric compounds youll find that for the most part youll need to account for the individual acid and.
The problem is HA - hydrolyses and dissociates at the same time and it is not obvious which of these processes will be be responsible for the final pH moreover it is very likely that pH can be attributed to some equilibrium between both reactions. Amphoteric Hydroxides Not all metal hydroxides behave the same way - that is precipitate as hydroxide solids. PH of amphoteric salts.
It might be because the amphoteric C₃N₄ NSs surface with carboxyl and amino groups possessed negative and positive charges in alkaline and acidic conditions respectively. H X 2 A A X 2 due reaction. The of the version that lost one proton is around 7 from the value of.
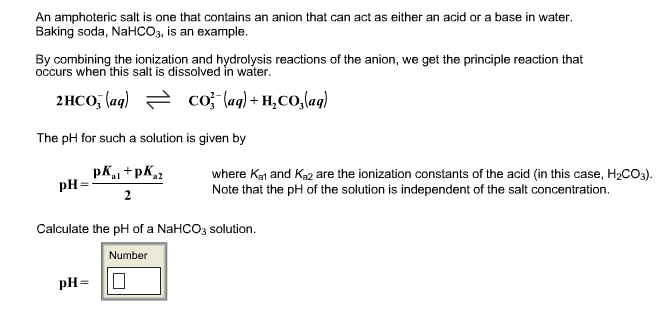
Function of pH with the phase separation of the polymer solution found to occur at a pH of. For example how could you calculate the pH of 0010 M NaHCO3. Solutions of both of these acids of concentrations around 1 mol dm-3 will have a pH of about 1.
Add 5 ml of 1 sample solution to 15 ml saturated bromine aqueous solution. The UV absorption spectra of the solution were continuously monitored in the titration vial by a fiber optic dip-probe. SLAA is typically supplied as clear yellow to amber-colored viscous aqueous solutions containing 2533 ww active SLAA and 69 ww residual NaCl.
Heat the mixture and observe the change of the precipitate. Hydroxide ions originated from water dissociation is much lower than concentration of the basic resp. The pH of a saturated lime ceCaOH2 solution is about 100.
The former equation assumes. Ionic Equilibrium-Lecture-6 for class XI About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features. The pH of the solution is independent of the concentration of the amphiprotic substance in the above example.
Once again you are unlikely ever to react this oxide with a base but you may well be expected to know how phosphoricV acid reacts with something like sodium hydroxide solution. Also some amphoteric copolymers were prepared via RAFT polymerization. However in this case how would you be able to tell whether it will accept or donate protons ie.
Whether it will be a base or acid. A saturated bromine aqueous solution can also be used to determine the type of amphoteric surfactants. The degree of ionization of P2VP in the diblock copolymer was studied as a function of pH with the phase separation of the polymer solution found to occur at a pH of.
The pH of the sample solution was adjusted to 18 with 05 M HCl before starting the titration and then the titration was done using 05 M KOH. I understand that the sodium ion would be a spectator and thus the HCO3 ion would contribute to the pH of the system. Amphoteric materials can be both positively and negatively charged depending on pH in solution relative to their point of zero charge PZC see Figure 1.
Also some amphoteric copolymers were prepared via RAFT polymerization. An amphoteric solution is a substance that can chemically react as either acid or base. However they did not report the thermo-responsive behavior of the amphoteric copolymers.
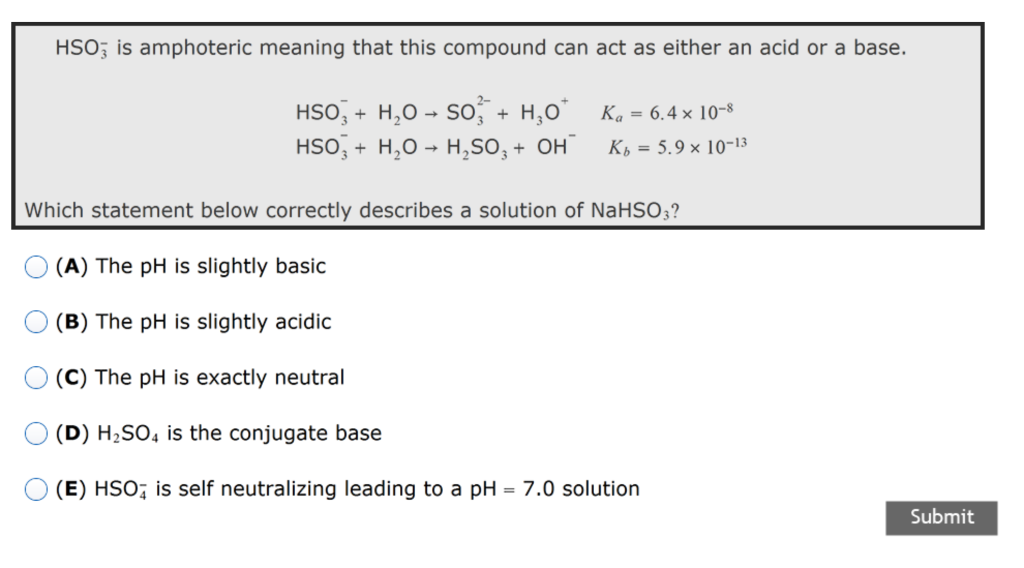
For example when HSO 4 - reacts with water it will make both hydroxide and hydronium ions. This justifies why its amphoteric. PART B In a monoprotic acid like HF all the F appears either as F or as HF two species.
In particular the Ch2R could be degraded completely within only 30 min in pH 11 solution. HSO_4- H_2O rightarrow SO_42- H_3O label11 HSO_4- H_2O rightarrow H_2SO_4 OH- label12. State your solution to the problem pH of resultant solution.

Calculating Ph Of Diprotic And Amphoteric Solutions Chemistry Stack Exchange

Polyprotic Acids And Amphoteric Salts Youtube

Calculating Ph Of Diprotic And Amphoteric Solutions Chemistry Stack Exchange

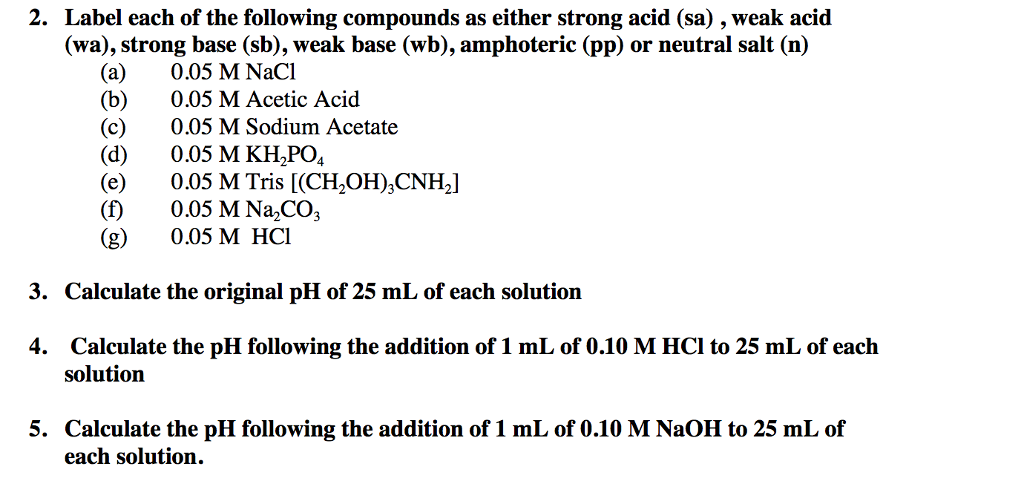
2 Protonated Salts These Are Usually Amphoteric Salts Which React As Acids And Bases For Example Nah2po4 In Water Would Show The Following Equilibria Ppt Download

Solved An Amphoteric Salt Is One That Contains An Anion T Chegg Com
Solved 5 1pt Circle Any Amphoteric Substances G Cn B Chegg Com
Solved An Amphoteric Salt Is One That Contains An Anion T Chegg Com

Section 16 2 Determining The Acidity Of A Solution 1 To Understand And Determine Ph And Poh 2 To Learn Methods For Measuring Ph Of A Solution Objectives Ppt Download
Welcome To Chem Zipper Com Amphoteric Salts Hydrolysis

Solved How To Calculate The Ph Of Different Solutions I Chegg Com
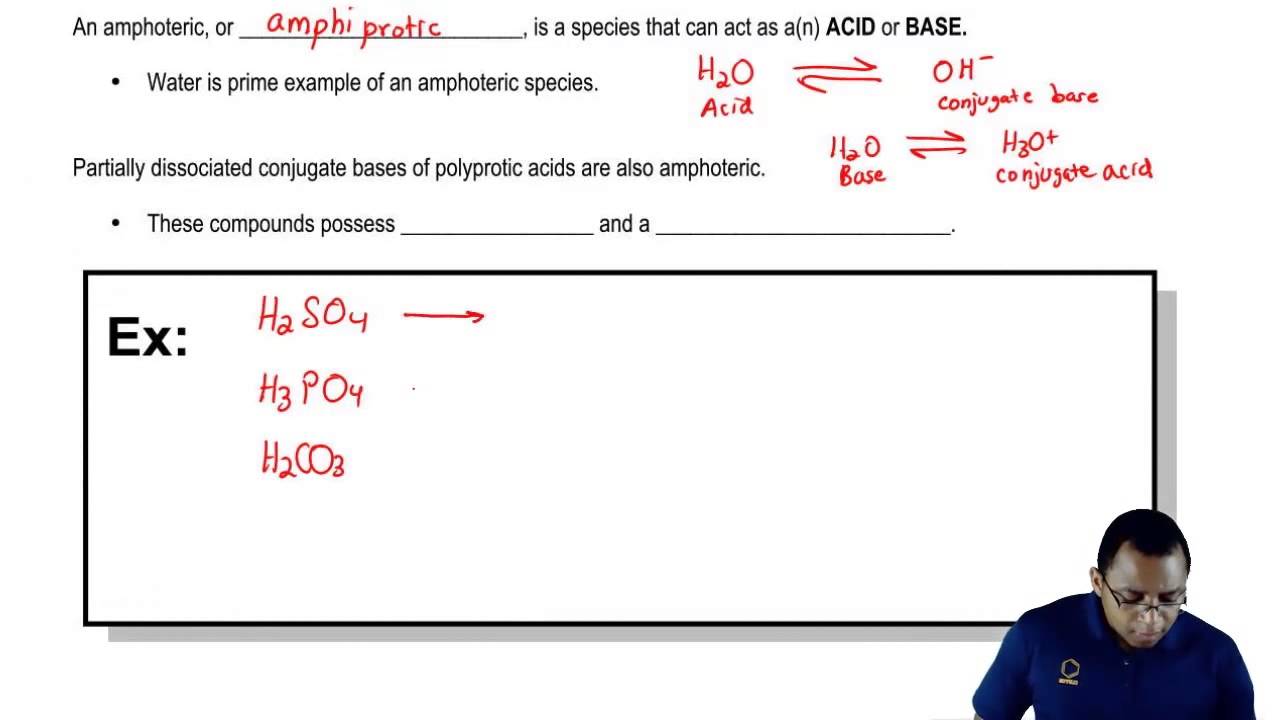
Understanding Amphoteric Species Youtube
Solved Hs03 Is Amphoteric Meaning That This Compound Can Chegg Com
General Questions On Acids 1 Define And Explain The Equilibrium Constant 2 Explain The Ionisation Of Water And The Constant Associated With This 3 Give Three Different Definitionsd Of Acids And Bases 4 Explain What Conjugate Acid Base Pairs Is 5