For example the density of water at its critical point T 374C P 2177 atm is 032 gmL about one-third that of liquid water at room temperature but much greater than that of water vapor under most conditions. - 3048794 dexterassault15 dexterassault15 17092020 Science Senior High School answered What is the critical pressure of water.
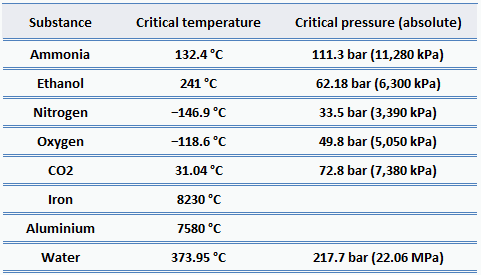
Critical Temperatures And Pressures For Some Common Substances
There is no change in the phase of water in the core.
What is the critical pressure of water. Critical Temperature K Critical Pressure MPa Critical Pressure atm Hydrogen H 332. Superheated steam can be used above the saturation limit. Note that at or above 374oC the critical temperature for water only water vapor exists in the tube.
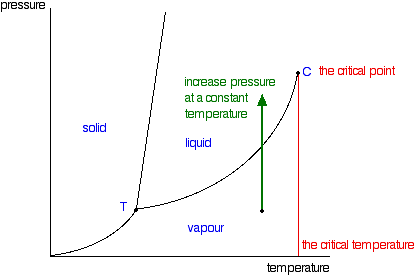
The critical temperature for a pure substance is the temperature above which the gas cannot become liquid regardless of the applied pressure. Ammonia NH 3 4055. The critical point is the intersection point of the critical temperature and the critical pressure.
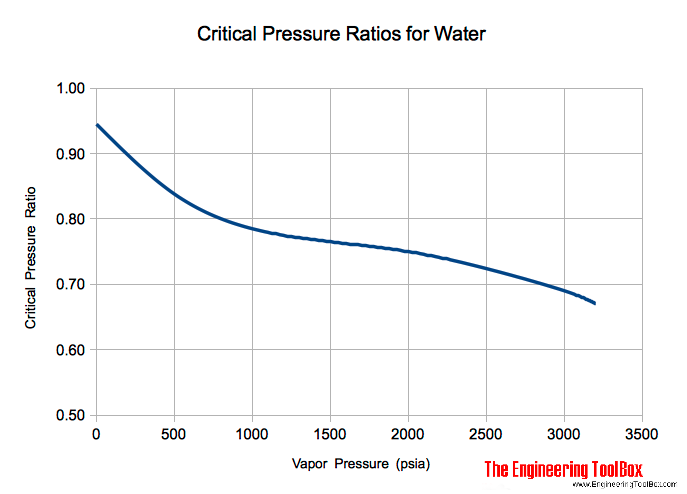
As for other substances water vapour pressure is a function of temperature and can be determined with. The end point of the pressure-temperature curve that designates conditions under which a liquid and its vapor can coexist. For example the critical pressure of hydrogen H 2 is approximately 13 MPa ie.
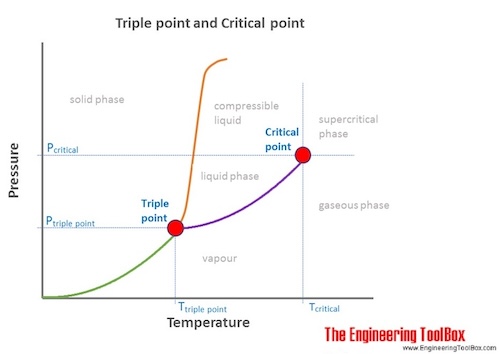
Critical pressure is the pressure below which it is not possible to carry out condensation of gas at a critical temperature. In thermodynamics a critical point or critical state is the end point of a phase equilibrium curve. At higher temperatures the gas cannot be liquefied by pressure alone.
The vapour pressure of water is the pressure at which water vapour is in thermodynamic equilibrium with its condensed stateAt higher pressures water would condenseThe water vapour pressure is the partial pressure of water vapour in any gas mixture in equilibrium with solid or liquid water. Critical pressure and temperature of water are 220 bar and 37314 C as steam water is a valuable heating agent below 200 C where the saturation pressure is about 24 bar. Critical pressure is Pcr 2209 MPa.
NH 3 corresponds to 1113 atm or 11280 kiloPascals. Cl corresponds to 76 atm. Boilers in conventional power plants have utilized once-through supercritical steam cycles since the mid-1950s.
For water the critical pressure is very high 21775 atm. Critical pressure is Pcr 2209 MPa. Propane C 3 H 8 370.
What is the critical pressure of water. For a pure substance the critical pressure is defined as the pressure above which liquid and gas cannot coexist at any temperature. The critical pressure of ammonia chemical formula.
At pressures above the critical pressure properties of water in the reactor change gradually and continuously from those we ordinarily associate with a liquid high density small compressibility to those of a gas low density large compressibility without a phase change. Carbon dioxide CO 2 3042. The critical pressure of chlorine symbol.
At the critical temperature the. Critical Pressures of Some Common Substances The critical pressure of water corresponds to 2177 atm or 22060 kiloPascals. At the critical point defined by the critical temperature T.
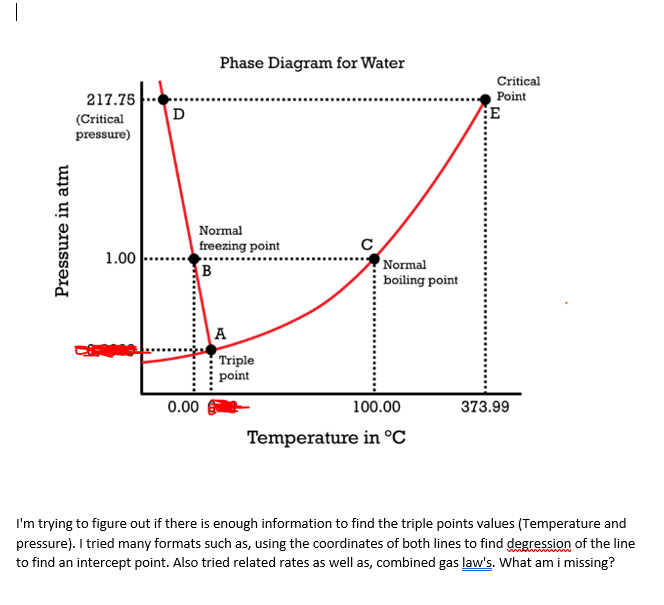
Once-through designs are more appropriate for supercritical operation because there is no phase change from water to. Critical point is where vapor and liquid are indistinguishable and triple point is where ice water and vapor coexist in thermodynamic equilibrium Water vapor pressure of 21775 atm 22064 bar 22064 MPa 32001 psi Temperature of 647096 K 373946 C 705103 F Critical point density. Critical Point of Water.
The phase diagram of water is a pressure-temperature diagram for water that shows how all three phases solid liquid and vapor may coexist together in thermal equilibrium. The transition between a liquidgas mixture and a supercritical phase is demonstrated for a sample of benzene in Figure PageIndex1. At pressure that is higher than the critical pressure of water water is in special state that is known as supercritical fluid state.
Every substance has a critical temperature. At pressure that is higher than the critical pressure of water water is in special state that is known as supercritical fluid state. On the other hand physical properties such as density.
The pressure required to liquify a substance vapor at its critical temperature. Critical pressure is specific for a given substance. The critical pressure of water is 3206 psia.
What Is Critical Point Of Water Quora

Critical Point Chemistry Libretexts

Critical Pressure Of Water Nuclear Power

Triple Point Of Water Analogy Fine Tuned Intensive Variables Critical Download Scientific Diagram
Solved Phase Diagram For Water Critical Point 217 7 Chegg Com

Critical Point Thermodynamics Wikipedia

Phase Diagram For Water Chemistry For Non Majors

Chemistry Glossary Search Results For Critical Volume
What Is Critical Point Of Water Definition

The Liquid Vapour Critical Point In A Pressure Temperature Phase Download Scientific Diagram